Abbott Laboratories (NYSE symbol: ABT) is an American multinational healthcare and medical devices company based in Abbott Park, Illinois. Known for its medical device and pharmaceutical products, the company reported net sales of $43.1 billion in 2021.
The company’s market cap is approximately $191 billion. Per the company’s website, they employ 113,000 globally. In our LinkedIn search, we uncovered 95 data scientists at Abbott.
Potential, non-mature use cases of AI and machine learning include devices in diabetes care and glucose monitoring.
In this article, we’ll examine how Abbott has used AI applications in its business and industry through two unique use cases:
- Micrometer-level medical imaging: Abbott uses AI automation to provide physicians with a precise and measurable way to capture image and risk data in the arteries of the eye or heart.
- Predicting heart attacks: Abbott developed an ML algorithm to identify individuals at risk of a heart attack.
Use Case #1: Micrometer-level Medical Imaging
Optical coherence tomography (OCT) is a non-invasive imaging technique that captures micrometer-level, two- and three-dimensional images within the arteries using reflected light. At Abbott, OCT software is being used to supply practitioners with real-time data on the heart vessels both before and during heart stint surgery.
According to clinicians, traditional manual methods of measuring related optical and heart vessel data are time-consuming, relatively inaccurate, and delay clinical decision-making. As such, any method which improves upon manual processes is a potentially significant step forward to delivering better patient outcomes.
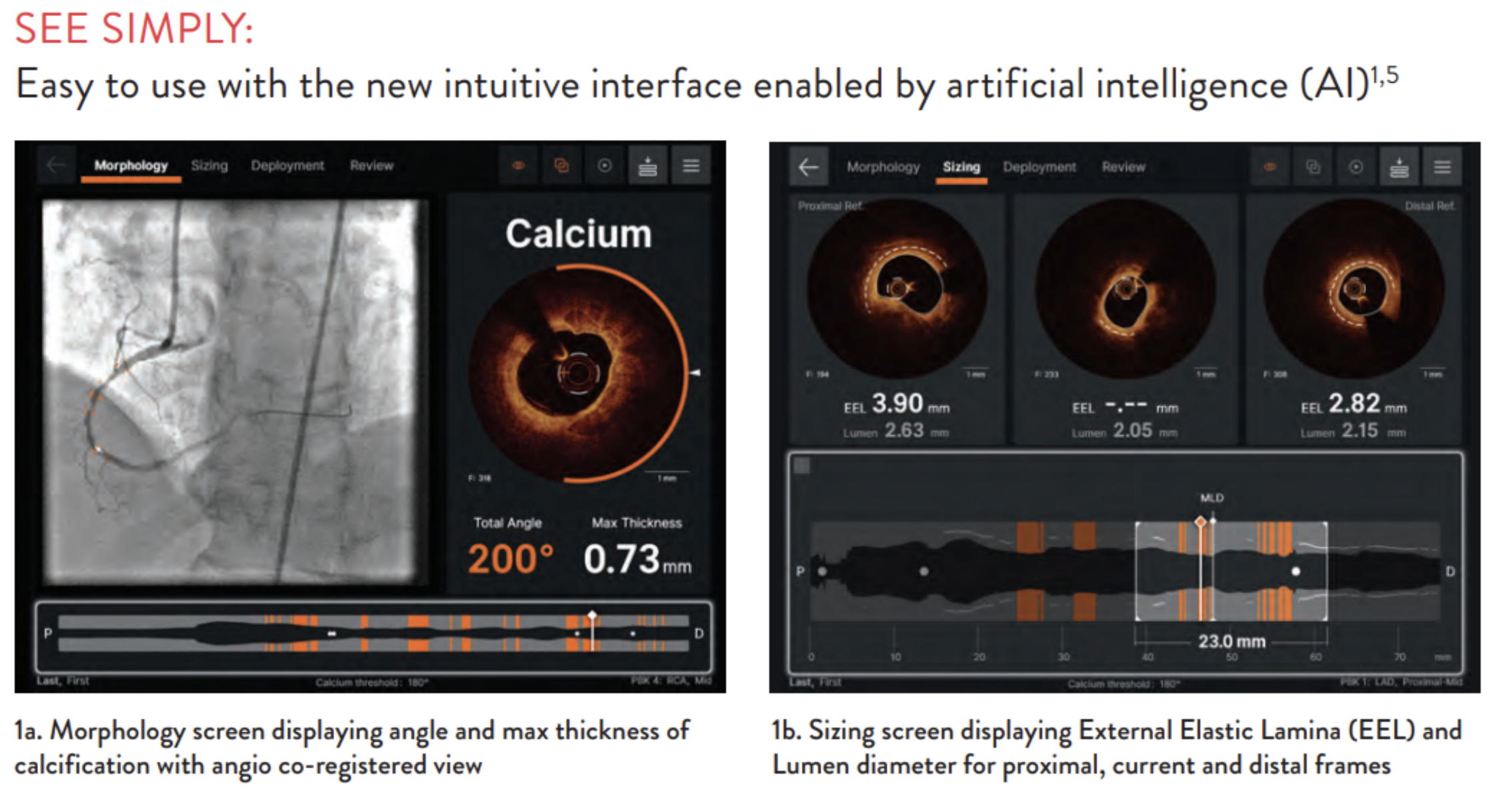
To overcome this problem, Abbott claims it has produced software called Ultreon. Abbott claims that clinicians see the inside of the heart and eye vessels before and during surgical procedures using an OCT imaging console equipped with the software.
The company states that Altreon can help healthcare providers by providing AI-enabled auto-detection of calcium levels and blood vessel diameters. Clinicians may use this data to make critical decisions in the operating room, such as determining the best placement of surgical objects, e.g., precisely where to insert stents in the heart vessel(s).
According to a study cited by Abbott, the software is trained on “multi-vendor, multi-scanner, and multi-hospital” anonymized patient data. Regarding the anatomical data, the model was trained using a territory map of a cardiac coordinate system.
After inserting the appropriately-sized catheter into the blood vessel, microsensors within the device capture image data, and algorithms “score” relevant measured data (depending on the procedure) against predefined risk criteria. The console displays the relevant image and data. Clinicians may then use this data to make decisions pre- or post-operation.
Among the output data:
- An orange “arc” that shows calcification angle (vessel walls are circular – 360 degrees – and this angle shows the extent to which the substance has built up around the wall)
- A Cross-sectional view displaying the AI-generated calcium thickness data
- Total values are calculated by the AI algorithm and displayed in real-time
Console with output data. (Source: Abbott)
Regarding business outcomes, Abbott has not released any financials pertaining to Ultreon. However, in an Abbott-sponsored study, the company claims that its OCT solution, when paired with Ultreon, results in 88 percent of physicians altering their treatment strategy. As a result, the company appears to be insinuating a high demand exists for the OCT platform in general and the Ultreon software in particular.
Use Case #2: Predicting Heart Attacks
According to Abbott, an estimated 840,000 Americans die annually from cardiovascular diseases. The company states that when testing for, diagnosing, and treating heart disease, time is of the essence. The company cites the following statistics:
- Around 47% of sudden cardiac deaths occur outside a hospital
- Cardiovascular disease (CVD) is the #1 cause of death globally
- 422.7 million CVD cases were reported globally in 2015
- By 2020, 31.7% of all deaths will have been from CVD
For these reasons, the company claims, it developed a “high sensitivity troponin-I blood test” capable of aiding doctors “in diagnosing heart attacks faster and more accurately” using its machine learning algorithms.
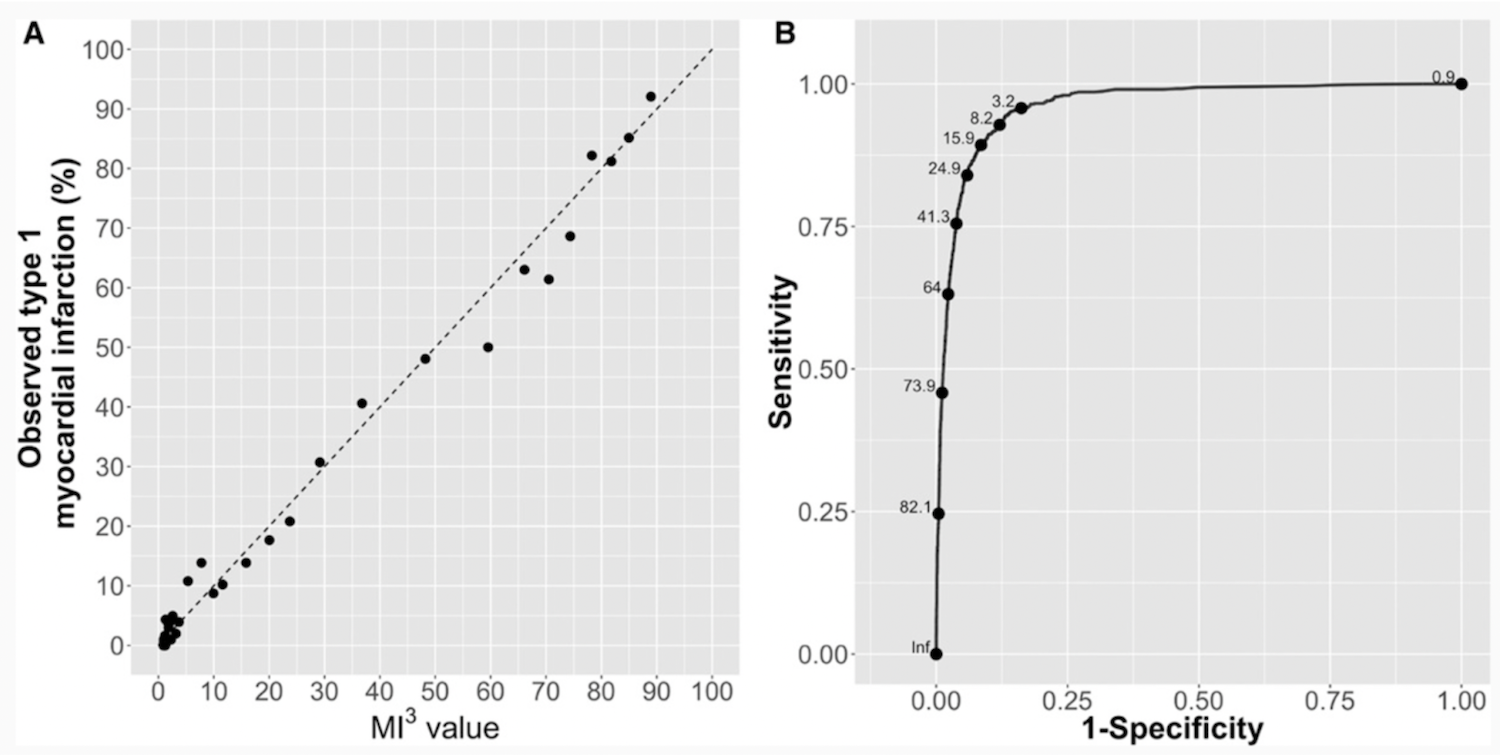
Abbott claims that a team of physicians and statisticians trained the product’s model using random patient data. The company states that its model identified the variables more predictive of a future cardiac event, including age, sex, troponin levels, and time of blood sample – and implemented these into its algorithm.
The success of the Abbott M3 model, signified by the promising correlation to “perfect calibration,” as represented by the dashed line in the “A” graph on the left. (Source: Abbott, Circulation.)
Abbott contrasts their solution with today’s common practice: a clinical assessment, electrocardiogram (EKG), and troponin blood tests at set intervals. The company states that its algorithm was designed to address two barriers for doctors trying to obtain more individualized information when diagnosing heart attacks. According to Abbott, these barriers are:
- International guidelines that do not account for personal details – age, sex, race, etc. – may impact test results.
- Guidelines that recommend troponin testing take place over fixed intervals. This recommendation is a potential problem, as it doesn’t consider age or sex.
Abbott claims that the efficacy of its proprietary test has been verified in a jointly published article published in the journal Circulation. Among the purported results:
- The test provides faster and more accurate detection of a current heart attack.
- More comprehensive probability analysis of whether someone has a heart attack “, particularly for those who entered the hospital within the first three hours.”
The article cites a 99% positive predictive value for low-risk patients and a 75% PPV for high-risk patients.
Regarding business outcomes, market research reports cite Abbott as the leading producer of cardiac biomarker products, under which the test mentioned above product falls. Percentage estimates of Abbott’s market share in this segment vary, but our research found an estimation range between 25 to 28%.