Pfizer (NYSE: PFE) is an American pharmaceutical and biotechnology corporation based in New York City. The company accrued $81.29 billion in revenue last year, currently holding rankings of 46th on the Fortune 500 and 49th on the Forbes Global 2000 lists.
Among the foremost initiatives in AI undertaken by the company was the highly publicized U.S. government response to the global pandemic that enabled three companies, including Pfizer, to produce effective COVID vaccines in record-breaking time.
The remarkable speed of vaccine production was the result, in part, not only of cutting red tape but also leveraging new AI tools like advanced computational modeling. However, applications of artificial intelligence at Pfizer began long before, and extend far beyond — COVID-19 vaccines today.
This article will take a closer look at three use cases showing how current AI initiatives support Pfizer’s immediate business objectives:
- Drug development and predicting regulatory queries: Pfizer is using machine learning in their drug development process to map the human immune system and, eventually, better predict government regulatory inquiries.
- Identifying symptoms of rare diseases: The pharmaceutical giant uses a novel prediction model — also derived from machine learning — to identify patients with rare diseases.
- Inventory prediction in vaccine supply chains: Pioneered by their COVID-19 vaccine initiatives, Pfizer tracks exact conditions of sensitive inventory through sophisticated data gathering to predict supply chain issues.
Beginning with our first use case, we find a machine learning-based example with wide application outside the pharmaceutical industry.
Use Case #1: Drug Development and Predicting Regulatory Queries
Companies are always trying to anticipate the response that their submissions will get from government regulators. And being able to anticipate rejections from regulators early stands to save a company like Pfizer billions in development costs.
Pfizer’s emphasis on the AI’s potential in public statements among their senior AI leadership signals what specific business objectives they see as enabled by using machine learning in the drug development process, particularly in the area of regulatory compliance.
In one such public statement, Pfizer Head of Information Management Boris Braylyan conveyed aspirations that AI could eventually help Pfizer “predict what queries regulators are likely to come back with. We may then be able to improve our submissions by predicting in advance what regulators are likely to ask, and coming prepared with those answers ahead of time.”
By cutting down on an endless back-and-forth of submissions taking weeks, anticipating regulatory feedback saves not just money but time — perhaps even more precious for enterprises when dealing with regulators.
In our research, we were able to obtain a compelling view of the current state of AI capabilities in Pfizer’s drug development process. While Pfizer has not quite achieved the level of enterprise AI integration necessary to predict government regulation queries, we can observe the large-scale data platforms Pfizer is putting into place to achieve such capabilities eventually.
According to news reports available on Pfizer’s website, the company uses machine learning throughout its drug development process. Among the most prominent of these efforts is a partnership with CytoReason to build a simulated model for the immune system or, as the company describes it, “a weather map for disease.”
The model is constructed in a two-step process:
- Enough data is taken from human blood required to conduct single-cell sequencing, a process that lets Pfizer scientists compare cells directly.
- CytoReason’s platform uses that data to train its model to trace deeper insights.
These insights include:
- Finding new biological targets for a disease.
- Understanding why certain populations respond to a drug.
- Predict conditions than can be treated using an existing drug.
In a use case depicting that final insight, Pfizer cites CytoReason’s platform:
- Helped their scientists understand the best application of an experimental compound that interferes with CCR6, a protein necessary for fighting autoimmune diseases.
- Traced patterns in molecular bindings between variations of a drug, proving which was more likely to perform for selection in clinical trials.
- Verified which existing diseases can benefit from a CCR6 inhibitor.
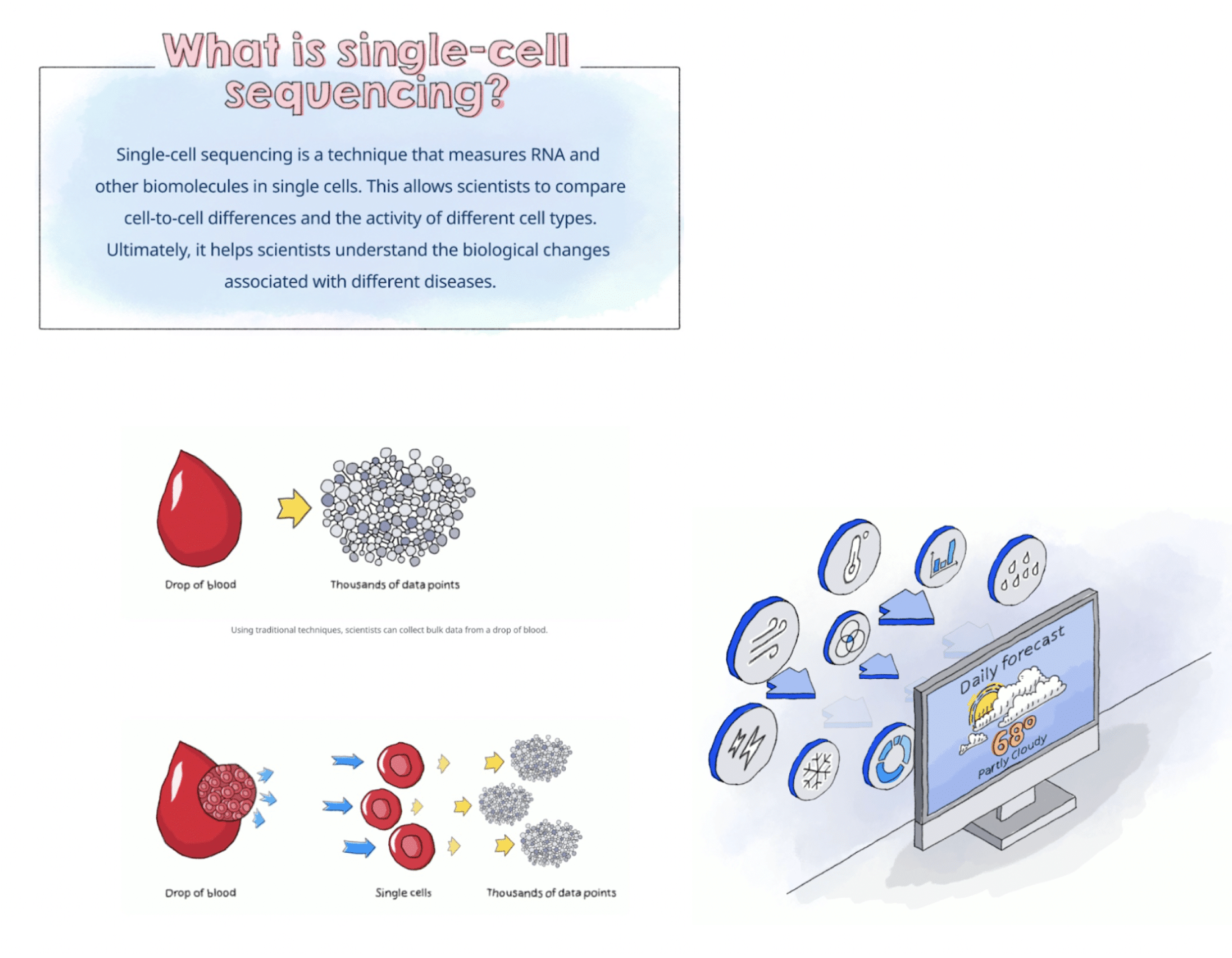
(Source: Pfizer)
By successfully replicating the human immune system in a computational model, as the company claims, Pfizer cannot only help forecast medical conditions in patients but aspires to one day “uncover novel medicines and identify which patients will benefit most from new treatments, potentially helping to speed up and reduce the costs of developing new drugs.”
It is also telling that the announcement also describes the model as a “platform”, which shows that the project represents a long-term investment in AI development on behalf of Pfizer and the product of a recent digital transformation in the company.
Earlier this year, Pfizer announced an expansion of their partnership with CytoReason in the areas of machine learning in their drug development process.
At the moment, there appears to be no public documentation yet regarding the outcomes of these initiatives on end users outside the laboratory. Much of Pfizer’s marketing and press materials cited in this article discuss AI capabilities in aspirational capacities, and showcase platforms – like the immune system model – that are very much in the early stages of development.
The long-term strategic thinking necessary to develop transformational ROI means that KPIs and outcomes for these technologies must be equally long-sighted. It might be the case – especially with a project seeking to accomplish nothing less than a computer simulation of the entire human immune system – that we’re still some years away from results Pfizer might be willing to share in a public forum.
It is also important to place these efforts in the broader context of two major factors:
- Enterprise AI adoption processes and their challenges at large, particularly for a legacy pharmaceutical firm like Pfizer.
- Challenges presented by the simultaneous global pandemic did not categorically help to hasten the early adoption process.
Use Case #2: Identifying Symptoms of Rare Diseases
If pharmaceutical companies are able to tangibly advance outcomes in rare conditions through their initiatives, not only does it help serve their bottom line and advance product lines, but also helps maintain public trust. Such public trust is especially precious to a company with nearly inextricable ties to the healthcare infrastructure of the United States.
Last year, Pfizer announced a successful initiative pioneering the use of predictive analytics to identify rare diseases like transthyretin amyloid cardiomyopathy (often abbreviated in scientific literature as ‘ATTR-CM’).
Specifically, Pfizer developed a novel machine learning model validated with patient-level data sets large enough to identify symptoms of such a rare disease – as the company has done with many similar studies cited robustly in relevant life sciences and AI-related academia.
When asked what the ATTR-CM initiative means for AI’s impact on diagnostics, Dr. Sanjiv J. Shah of Northwestern University remarked on the technology’s potential for correct diagnoses at earlier stages of the disease.
“Which is critical for a progressive condition like ATTR-CM, with a life expectancy of only two to three-and-a-half years on average after diagnosis if untreated,” Dr. Shah emphasized in his statement.
The study, published in cooperation with the highly respected Nature Communications journal, verified the following results for Pfizer:
- 87% accuracy in predicting wild-type transthyretin amyloid cardiomyopathy (wtATTR-CM) heart failure patients compared to other comparable patients.
- A systematic framework built from the first algorithm of its kind to leverage artificial intelligence machine learning across medical claims data.
- Using that systematic framework, Pfizer developed EstimATTR, a platform for estimating the probability that a hypothetical patient with heart failure may have wtATTR-CM.
The EstimATTR platform was revealed later in 2021. Following instructions in this manual, patients and their doctors can use the dashboard depicted in the screenshot below to plugin the following inputs:
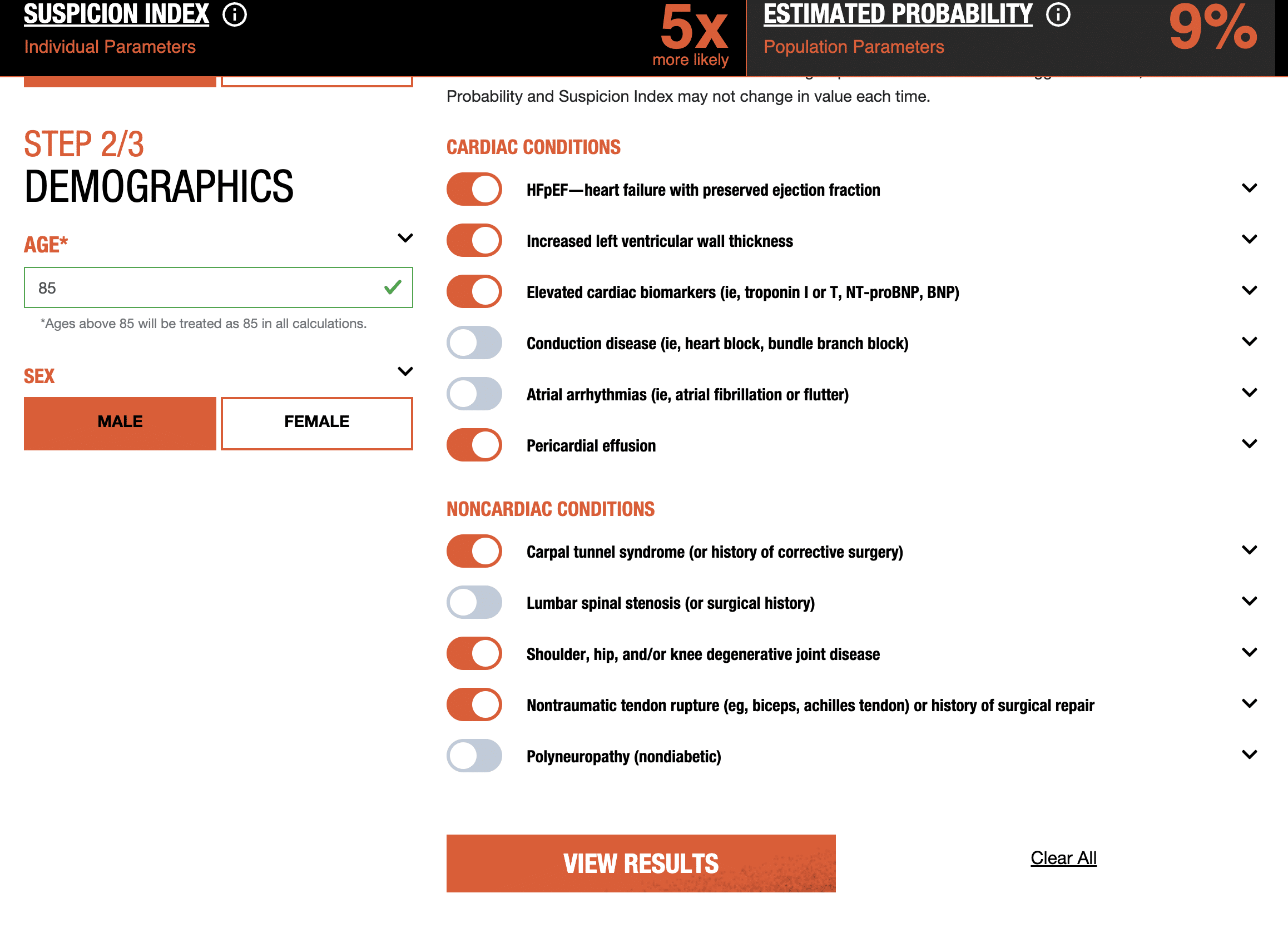
(Source: Wild Type EstimATTR-CM)
The inputs are based on symptoms identified in the Nature Communications joint study by Pfizer’s AI-enabled analytics.
As this tool was revealed only last year and just a few months after the results of the study were published, there are currently no published results of outcomes for end users that are publicly available at the moment – from Pfizer or otherwise. The only other public statements from the company with regard to ATTR take the form of awareness campaigns.
Looking at the entire lifespan of AI capabilities demonstrated in this use case through Pfizer’s ability to develop tools for identifying symptoms of rare diseases, we can conclude the company is currently able to:
- Use AI capabilities to identify the symptoms of rare conditions previously undetected in research.
- Build simple data collection and screening tools, based on those symptoms, to help patients identify rare diseases earlier and with greater accuracy.
- Launch these screening tools within months of publishing the research confirming the use of AI identifying symptoms.
Use Case #3: Inventory Prediction in COVID-19 Supply Chain Compliance

Shipments of the Pfizer-BioNTech COVID-19 vaccine from the U.S. to Bangladesh. (Source: U.S. Embassy Dakha)
In 2019, Pfizer founded their Digital Operations Center to handle the enterprise-wide adoption of these technologies, such as AI capabilities.
Simultaneously, the onset of the global pandemic presented both challenges and opportunities for long-term technological adoption. Development of Pfizer’s mRNA COVID-19 vaccine with partner BioNTech required strenuous compliance with evolving government oversight and regulations throughout development and production.
In the process, Pfizer developed AI capabilities in areas outside of simple compliance, such as inventory prediction, to meet regulatory standards. A partnership with Controlant was able to guarantee conditions of vaccine and component delivery across supply chains.
In other marketing literature describing how AI capabilities ensure Controlant’s offering of “cold chain as a service,” their platform improves supply chains using machine learning, data analytics, and automation – as they have for the Pfizer-BioNTech vaccine – in four distinct capacities:
- Reducing Risk: Collecting volumes of data from IoT-connected devices and sensors in the cold chain to predict better performance, identify risk areas, and provide better control.
- Improving decision-making: Provide management with accurate logistics insights based on real-time information.
- Improving partnership selection: Helping clients make partnership decisions for the integrity of their supply chain based on data analytics and machine-guided insights.
- Improve CX: Data analysis of real-time tracking information lets Controlant provide customers with more accurate estimates for shipment arrival.
Together, the companies pioneered a new type of data tracking that could handle GPRS signal functionality, and send large amounts of up-to-date information at once. Monitoring devices came preassembled in shipping containers and with simple instructions for ease of use among handlers on the factory floor.
Thanks to Controlant’s cloud capabilities, conditions of vaccine components were able to be tracked in real time. In a wide-ranging interview with McKinsey, Chief Digital and Technology Officer at Pfizer Lidia Fonseca said data like that collected on the vaccine supply chain is being collected and analyzed by the company’s industry-first digital operations center to “predict issues and make adjustments” to the entire supply chain in real-time.
Testimony from an anonymous Pfizer engineer told Emerj researchers that Controlant’s real-time data capabilities, in particular, greatly improved outcomes in workflows for those tasked with ensuring the compliance of COVID-19 supply chains with regulations. Unlike Pfizer’s other partnerships with supply chain integrity vendors like Sensitech – Controlant’s real-time data capabilities in tracking shipments marked the first such partnership of its kind with Pfizer.
In terms of ensuring vaccine delivery, a statement released earlier this year from Controlant assessing their larger participation in Operation Warp Speed found their partnership with Partnership yielded 99.999% of Pfizer-BioNTech vaccines successfully reaching their receiving sites. It should be telling that the excursion rate is the only figure mentioned in the release with regard to concrete outcomes.
While the pandemic is far from over from a global perspective, the lack of information in the statement with regards to the partnership’s involvement in delivering components, and the lack of corresponding press materials from Pfizer are somewhat telling.
For such a publicly visible initiative with huge public investment, only touting a 0.0001% excursion rate over a year’s worth of sensitive shipments leads us to suspect that the results of the partnership in this use case are still being assessed by their respective stakeholders.
Elsewhere in press statements from Controlant, the company described their COVID-19 “cold chains” as run amok with materials shortages and production challenges.
A possibility could be that the data on the results that these stakeholders have cannot yet demonstrate the definitive difference that inventory prediction capabilities had on decreasing risk in an already highly disrupted supply chain. However, such circumstances would lend great credibility to the integrity of their extremely low excursion rate, which they’re willing to publicize for the time being.














